Class 2 Medicines Recall: Lercanidipine HCl 20mg Tablets, Recordati Industria, EL(25)A/17
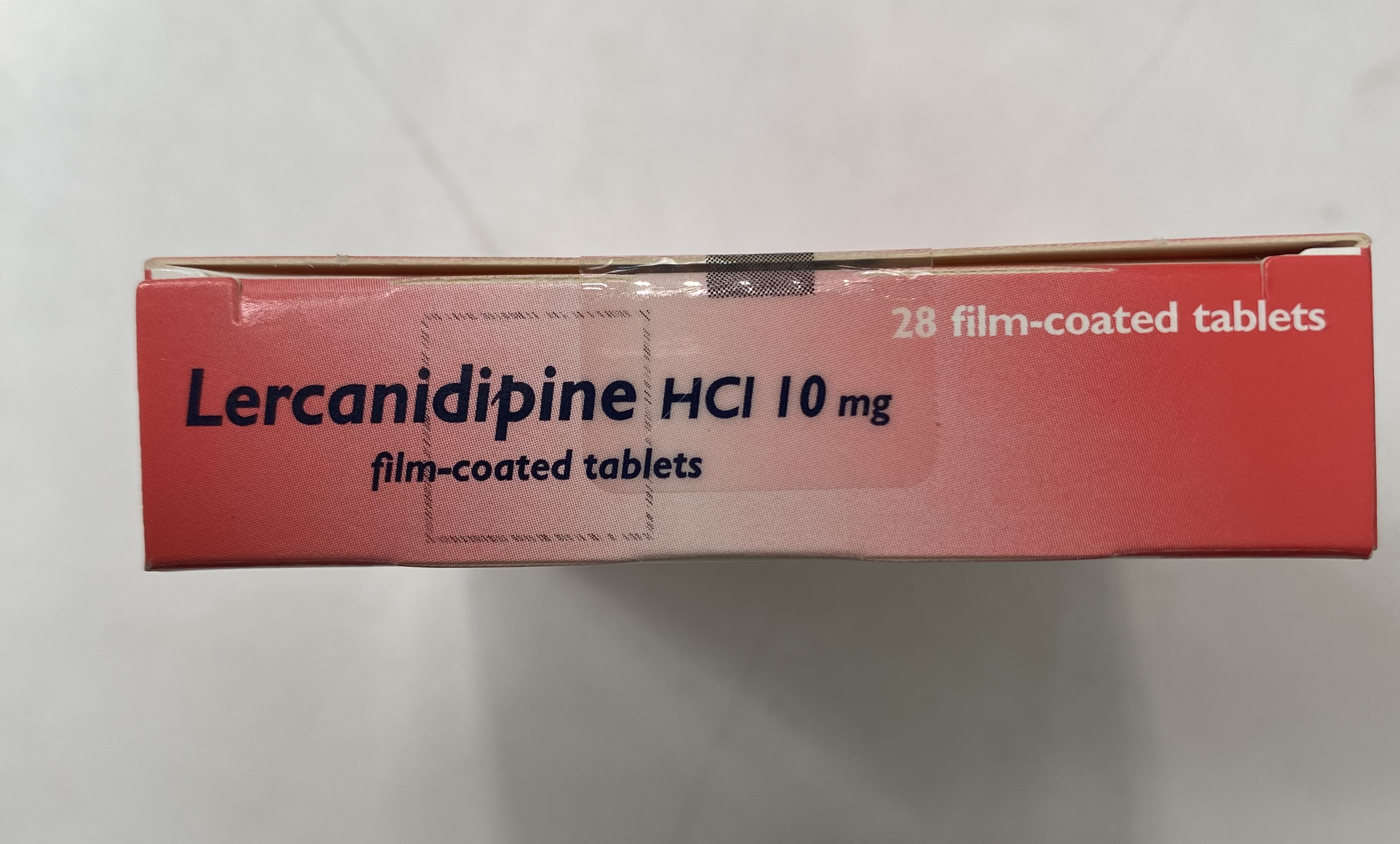
Recordati Pharmaceuticals Limited has informed the MHRA of an error in the strength of the product printed on some of the faces (sides) of the product carton. The error is limited to one batch of Lercanidipine HCl 20mg Tablets. The packs are incorrectly labelled as 10mg on some sides of the product carton when they are 20mg tablets.
DMRC reference number
DMRC Ref: 35334038
Marketing Authorisation Holder
Recordati Industria Chimica e Farmaceutica S.p.A. Via Matteo Civitali, 1 - 20148 Milan, ITALY.
Medicine Details
Lercanidipine HCl 20mg Tablets
PL: 04595/0011
Active ingredient: lercanidipine hydrochloride
SNOMED code: 21857911000001106
GTIN: 8057742821983
Affected Lot Batch Numbers
| Batch No. | Expiry Date | Pack Size | First Distributed |
|---|---|---|---|
| MD4L07 | 01/2028 | 28 | 10/04/2025 |
Background
Recordati Pharmaceuticals Limited has informed the MHRA of an error in the strength of the product printed on some of the faces (sides) of the product carton. The error is limited to the batch of Lercanidipine HCl 20mg Tablets listed in the table above. The packs of 20mg tablets are incorrectly labelled as 10mg on some sides of the product carton. The correct strength (20mg) is printed on the top of the carton and on the blister strips. Recordati Pharmaceuticals Limited is initiating a recall of the specified batch as a precautionary measure.

Advice for Healthcare Professionals:
Stop supplying the above batch immediately. Quarantine all stock and return it to your supplier using your supplier’s approved process. This medicine is being recalled as a precautionary measure. Pharmacists who have been supplied this batch and suspect they have dispensed the tablets to patients should contact the patient immediately to make them aware of this error. Please see ‘advice for patients’ section below.
Advice for Patients:
Patients who have been prescribed 10 mg tablets and have received tablets with this batch number (printed on the foil of the blister strips) should contact your pharmacist or GP immediately. In the event that the GP or pharmacist cannot be reached, please call NHS 111 for advice on continuing your medication. In the event you cannot speak to a healthcare professional before you are due to take your next dose:
- verify the strength of the tablets is 20 mg from the information on the foil of the blister strips
- remove one tablet from the blister as normal
- locate the break line on the tablet
- snap the tablet in half across the break line and take half of the tablet. This is permitted for the 20mg tablets and is in line with information included in the patient information leaflet (where it states ‘The tablet can be divided into equal doses’).
Patients who have been prescribed 20 mg tablets should verify the strength of the tablets by checking the information on the foil of the blister strips prior to taking the tablet. Continue to take the tablets as prescribed by your doctor.
Do not stop taking your medicine without consulting your healthcare provider. Patients who are concerned about the strength of the medication they have received should check it with their dispensing pharmacy.
Patients concerned they may have accidentally taken a higher dose of the medication than they were prescribed should seek medical attention.
Patients who experience adverse reactions or have any questions about the medication should seek medical attention. Any suspected adverse reactions should also be reported via the
Additional information:
For all medical information enquiries and information on this product, please email medinfo@recordati.co.uk, or telephone +353 (0)87 6245669.
For stock control enquiries please email customerservice@recordati.com, or telephone 01491 576 336.
Recipients of this Medicines Notification should bring it to the attention of relevant contacts by copy of this notice. NHS regional teams are asked to forward this to community pharmacists and dispensing general practitioners for information.
Yours faithfully
Defective Medicines Report Centre
10 South Colonnade
Canary Wharf
London
E14 4PU
Telephone +44 (0)20 3080 6574
Download document